Abstract
Background
Myelodysplastic syndrome (MDS) arises from and is perpetuated by malignant stem cells. Curative therapy requires eradication of this population, but this is not achieved with currently available medical therapies. MDS stem cells possess unique molecular properties that can be therapeutically exploited, but in general these strategies have not been explored clinically. Our recent in vitro studies showed that combination treatment with the protein synthesis inhibitor omacetaxine (oma) and the hypomethylating agent (HMA) azacitidine (aza) efficiently targeted MDS stem cells with minimal impact on normal stem cells. Based on these findings we conducted a Phase 1 clinical trial for MDS patients with concomitant oma + aza (NCT03564873).
Methods
Patients were eligible if they had newly diagnosed MDS with ≥5% blasts. Aza was given at 75 mg/m 2 IV days 1-7 of a 28-day cycle; oma was given subcutaneously twice daily on days 1-7 of a 28-day cycle with 4 dose cohorts (0.75, 1.0 and 1.25 mg/m 2, and a de-escalation cohort of 0.5 mg/m 2). Dose escalation was based on a 3+3 design. Bone marrow biopsies occurred at baseline and day 8 and 28 of cycle 1, and at the conclusion of odd-numbered cycles. The primary endpoint was the maximum tolerated dose (MTD) of oma with aza. Responses were assessed according to 2006 IWG criteria with hematologic improvement (HI). Using pre- and post-treatment patient samples, advanced single cell techniques including mass cytometry and antibody-based single-cell RNA sequencing (CITE-Seq) were used to elucidate mechanisms of sensitivity and resistance. High sensitivity DNA sequencing techniques were used to understand clonal evolution and detect measurable residual disease (MRD).
Results
Nine patients were required to complete phase 1. See Table 1 for baseline characteristics. Two patients in cohort 1 experienced dose limiting toxicity (DLT) (grade 3 hypoxia and grade 3 respiratory failure). De-escalation to cohort 0 resulted in one DLT event (grade 4 GI bleed) among the six patients treated in this cohort. The MTD was established as 0.5 mg/m 2 oma days 1-7. Adverse events are detailed in Table 2. Median number of cycles was 2 (1-3). 8/9 patients achieved a marrow CR; 5 had HI. 6 patients proceeded to transplantation and none have relapsed with median time from transplant of 772 (183-1158) days. Median response duration is 587 days (29-938). 5 remain alive.
MRD negativity, as measured with droplet digital PCR (sensitivity up to 0.1%), was achieved in 2 out of 4 patients measured. Both MRD negative patients had complete clearance of mutated splice factor genes; we previously showed no ability to clear these mutations (0/13 patients) using venetoclax+aza in AML (Nature Medicine (2018) 24:1859-1866).
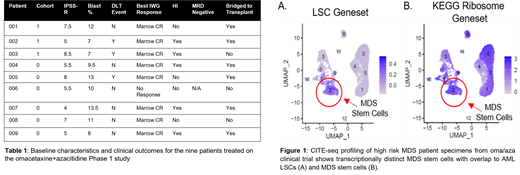
Baseline analysis of patient specimens by single cell transcriptomics demonstrates a distinct population of MDS stem cells (Figure 1A). This subset has overlapping transcriptional properties with known AML stem cell profiles as highlighted by increased geneset expression. Within the stem cell compartment, we observed up-regulation of protein synthesis pathways (Figure 1B) (e.g. KEGG ribosome pathway in MDS stem cells), indicating that oma/aza treatment may specifically target the malignant stem cell population. Subsequent studies are investigating the use of CITEseq as a means to evaluate drug response, which will provide very high resolution analyses of transcriptional signatures in MDS patients. By specifically monitoring eradication of MDS stem cells during the course of treatment, it should be possible to predict therapeutic efficacy and response duration while elucidating genes that correspond to oma/aza sensitivity and resistance.
Conclusions
Phase 2 of this study is ongoing; 23 newly-diagnosed high risk MDS patients will be enrolled with a primary endpoint to determine the overall response rate. In addition a ten-patient HMA failure expansion cohort will be enrolled to determine preliminary toxicity and efficacy assessments. Correlative studies will seek to determine the impact of this regimen on the MDS stem cell population and the mechanism of this impact.
McMahon: Takeda: Membership on an entity's Board of Directors or advisory committees. Smith: Syros: Research Funding; Kura: Research Funding; Argenx: Research Funding. Pollyea: Foghorn: Honoraria; Gilead: Consultancy, Honoraria; Celgene: Honoraria; Bristol Myers Squibb: Honoraria; Genentech: Consultancy, Honoraria; Jazz: Honoraria; AbbVie: Consultancy, Honoraria, Research Funding; Amgen: Honoraria; Astellas: Honoraria; Aprea: Honoraria; Karyopharm: Consultancy, Honoraria; Kiadis: Honoraria; Novartis: Consultancy, Honoraria; Syndax: Honoraria; Syros: Consultancy, Honoraria; Takeda: Honoraria; Teva: Research Funding.
Omacetaxine for MDS

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal